Freshwater scarcity, one of the most critical global challenges of our time, poses a major threat to economic growth, water security, and ecosystem health. The challenge of providing adequate and safe drinking water is further complicated by climate change and the pressures of economic development and industrialization. The public and industrial sectors consume substantial amounts of freshwater while producing vast quantities of wastewater. If inadequately treated, wastewater discharge into the aquatic environment causes severe pollution that adversely impacts aquatic ecosystems and public health. Recovery and recycling of wastewater has become a growing trend in the past decade due to rising water demand. Wastewater reuse not only minimizes the volume and environmental risk of discharged wastewater, but also alleviates the pressure on ecosystems resulting from freshwater withdrawal. Through reuse, wastewater is no longer considered a “pure waste” that potentially harms the environment, but rather an additional resource that can be harnessed to achieve water sustainability. Zero liquid discharge (ZLD) is an ambitious wastewater management strategy that eliminates any liquid waste leaving the plant or facility boundary, with the majority of water being recovered for reuse. ZLD obviates the risk of pollution associated with wastewater discharge and maximizes water usage efficiency, thereby striking a balance between exploitation of freshwater resources and preservation of aquatic environments. Achieving ZLD, however, is generally characterized by intensive use of energy and high cost. As a result, ZLD has long been considered not viable and has been applied only in limited cases. In recent years, greater recognition of the dual challenges of water scarcity and pollution of aquatic environments has revived global interest in ZLD. More stringent regulations, rising expenses for wastewater disposal, and increasing value of freshwater are driving ZLD to become a beneficial or even a necessary option for wastewater management. The global market for ZLD is estimated to reach an annual investment of at least $100−200 million, spreading rapidly from developed countries in North America and Europe to emerging economies such as China and India. Although ZLD holds great promise to reduce water pollution and augment water supply, its viability is determined by a balance among the benefits associated with ZLD, energy consumption, and capital/operation costs. Therefore, it is imperative to understand the drivers and benefits that make ZLD a realistic option. Incorporating new technologies, such as emerging membrane-based processes, provides opportunities to reduce the associated energy.
Zero Liquid Discharge or not to be?
The demand for Zero Liquid Discharge often comes with the company policy or in order to cope with unstable water supply. Zero Liquid Discharge aims at the maximum possible reuse of wastewater – but is it also economical? With a fraction of the costs, already large quantities of wastewater can be recycled. The last few percent water recovery increase the costs for such a system considerably. This is also because the treatment residues, the brine, need to be disposed of expensively. As a result, the reclaimed water may get more expensive than fresh water. With an alternative solution with a combination of elimination and separation process steps, a better balance between ecology and economy can be achieved: The industrial wastewater can be treated to a level that still allows the concentrate to be discharged cheaply in the sewers. Typically the clean majority of the treated water can be used for internal processes.
■ CONVENTIONAL ZLD SYSTEMS
Thermal ZLD Systems
Early ZLD systems were typically based on a series of thermal processes. In such systems, the feed wastewater undergoes a pretreatment step that reduces scaling potential, and is then concentrated sequentially by two core elements; a brine concentrator and a brine crystallizer (or an evaporation pond). The distillates generated by the brine concentrator and crystallizer units are reused as clean product water, whereas the solids produced are either stored (in evaporation ponds), further processed for landfill disposal, or reused as valuable byproducts. Despite their limitations, brine crystallizers or evaporation ponds are still indispensable for ZLD processes. Therefore, the focus of ZLD technology has been on reducing the volume of concentrated brine entering the brine crystallizers or evaporation ponds.
Reverse Osmosis
RO, a well-established, pressure-driven desalination technology with excellent energy efficiency compared to thermal desalination, has been incorporated into ZLD operation to lower energy consumption. Unlike thermal processes, RO does not require product water to undergo phase transition to achieve separation, thereby eliminating irreversible losses associated with evaporation and condensation in thermal processes. The energy consumed by the RO stage in seawater desalination at 50% recovery is as low as ∼2 kWhe/m3 of product water, which is significantly lower than that by brine concentrators and crystallizers. A smaller amount of energy is required when treating feedwater with lower salinity than seawater (e.g., brackish water RO, BWRO). In addition, the modular nature of membrane-based technologies provides further versatility in adapting RO into wastewater treatment facilities. As a result, RO can be used to pre concentrate the feed water prior to the more energy-intensive thermal processes, increasing both energy and cost efficiencies of ZLD systems.
EMERGING MEMBRANE-BASED ZLD TECHNOLOGIES
Three membrane-based processes
• ED (Electrodialysis)
• FO (Forward osmosis) and
• MD (Membrane distillation)
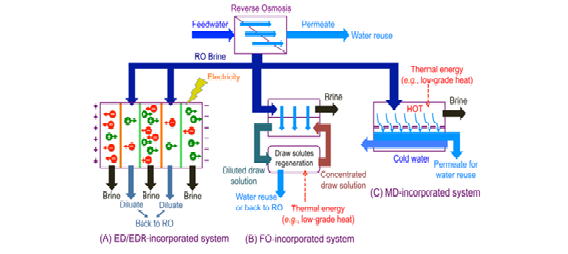
Emerge as alternative ZLD technologies to brine concentrators to further concentrate the wastewater after the RO stage. The produced brine from these processes serves as a feed to the crystallizer or evaporation pond. A schematic illustration of ZLD systems incorporating these technologies is shown in Figure below. Some of these technologies (i.e., thermolytic FO and MD) are hybrids of both thermal- and membrane-based processes. While the energy
input to these processes is thermal, membranes are the core separation components of these technologies.
- Electrodialysis
ED applies an electric potential as the driving force to remove dissolved ions through ion exchange membranes. In contrast to RO membranes that reject all ions, ion exchange membranes selectively permit the transport of counter ions but prevent the passage of co-ions. Cations move toward the negatively charged cathode by passing through cation-exchange membranes, whereas anions migrate in the opposite direction through anion-exchange membranes. As a partial desalination process, ED/EDR has been applied in combination with RO in several ZLD systems. Such systems achieved the dual function of extending the salinity limit of RO and reducing the energy consumption relative to brine concentrators. For example, Oren et al. demonstrated a pilot RO-EDR system for brackish water desalination with a water recovery of 97−98%.5 In that system, EDR concentrated the RO brine to a salinity of 100 000−200 000 mg/L prior to a side-loop crystallizer and wind-aided intensified evaporation. In another pilot study, EDR effectively removed hardness to reduce the scaling potential of saline basal aquifer water, thereby improving the subsequent RO recovery without chemical addition. The EDR brine could reach a salinity of 125 000 mg/L and was further concentrated by a brine crystallizer to approach ZLD. In both cases, the EDR effluent was further desalinated by RO or partially blended with RO permeate to attain a desired product water quality.
Forward Osmosis
Unlike hydraulic pressure-driven RO, FO utilizes an osmotic pressure difference to drive water permeation across a semi permeable membrane. In FO, water flows from the feed water to a concentrated draw solution with a higher osmotic pressure. The produced brine is sent to a brine crystallizer or an evaporation pond, whereas the draw solutes are separated from the desalinated water to regenerate the concentrated draw solution. Since the driving force in FO is osmotic pressure, FO can treat waters with much higher salinity than RO. When using FO to concentrate feed water beyond the salinity limit of RO, the osmotic pressure of diluted draw solution will surpass the bearable pressure limit of RO. Hence, in this case, draw solutes that depend on RO for regeneration (e.g., NaCl and MgSO4) will not be suitable. The development of thermolytic draw solutes, such as the ammonia−carbon dioxide (NH3/CO2), paved the way for FO incorporated ZLD systems. The NH3/CO2 draw solution generates very high osmotic pressure-driving forces and can be regenerated by low-temperature distillation. A recent pilot study demonstrated the application of FO with NH3/CO2 draw solution to concentrate produced water from the Marcellus shale region to an average salinity of 180 000 mg/L. Because the thermolytic NH3/CO2 draw solution decomposes at moderate temperature (approximately 60 °C at atmospheric pressure) low-grade thermal energy, including industrial waste heat and geothermal energy, can be utilized to regenerate the concentrated draw solution.
Membrane Distillation
MD is a thermal, membrane-based desalination process, in which a partial vapor pressure difference drives water vapor across a hydrophobic, micro-porous membrane. In MD, the feed water is heated and the resultant temperature difference between the hot feed water (typically 60− 90 °C) and colder permeate side creates a vapor pressure difference to drive the water vapor flux. The aqueous permeate can be in direct contact with the membrane (direct contact membrane distillation, DCMD). Alternatively, the water vapor can be collected on a condensation surface separated from the membrane, such as in air gap membrane distillation (AGMD), vacuum membrane distillation (VMD), or sweeping gas membrane distillation (SGMD). MD is more energy intensive than RO and ED/EDR, because water separation by MD requires liquid−vapor phase transition. The theoretical minimum energy of seawater desalination by single-pass DCMD with heat recovery and a feed temperature at 60 °C is 27.6 MJ/m3 of product water, which is much higher than that by RO with a typical recovery of 50% (3.8 MJ/m3 of product water).8 In practical use, DCMD was estimated to consume 143−162 MJ (40−45 kWht ) per m3 of product water for seawater desalination,76 and a comparable value of 80−240 MJ (22−67 kWht )/m3 of product water was reported for AGMD. However, this thermal-based energy consumption cannot be directly compared with the energy consumption of electricity-driven technologies (RO, ED/EDR, and MVC brine concentrators), because the efficiency of electricity generation from thermal energy varies with the quality (temperature) of the thermal energy. Compared to MVC brine concentrators with well-designed energy recovery devices, efficient heat recovery (e.g., use of heat exchangers or brine recycling) is critical to improve the energy competitiveness of MD.
ENVIRONMENTAL IMPACTS
Despite the main goal of ZLD to reduce water pollution and improve water sustainability, application of ZLD also results in unintended environmental impacts. One risk stems from the produced solid wastes. For example, solid wastes stored in evaporation ponds have raised concerns about their odors, potentially negative impact on wildlife, and risk of leakage. Similarly, solid wastes disposed in landfills may result in leaching of chemicals into groundwater. Accordingly, impervious liners and reliable monitoring systems are typically required to prevent potential contamination from solid wastes. As discussed earlier, ZLD consumes large amounts of energy, leading to significant emission of greenhouse gases (GHG). Some pretreatment methods, such as acidification followed by degasification, release CO2 from the feedwater into the atmosphere. For example, the application of ED in concentrating RO brine increases CO2 emission via both energy consumption and decarbonation for scaling control. Incorporating technologies with higher energy efficiency, such as RO, will significantly reduce the GHG emission. In addition, emerging ZLD technologies that can utilize low-grade or renewable energy (e.g., waste heat, solar energy, geothermal energy enable further reduction of GHG footprint of ZLD systems. Along with advances in improving the energy and cost efficiencies of ZLD technologies, particularly by incorporating membrane-based processes, ZLD may become more feasible and sustainable in the future.